Compounding sterile preparations: Onus or opportunity?
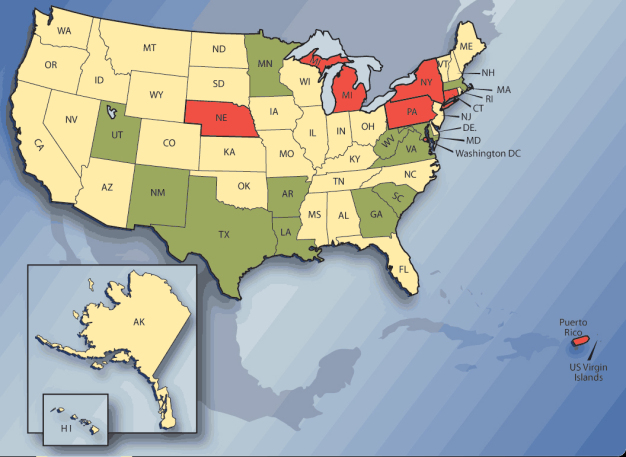
By Bruce Flickinger Early in ago more career, Mary Monk Tutor, PhD, spent many years as A home infusion pharmacist and for 12 years which A Joint Commission Home Care Surveyor. She remembers the time when home care patients were trained tons mix totally parenterally nutrition (TPN) formulations in their kitchens, and when nurses in hospital compounded intravenous solutions on countertops. � incoming goods did this into the industry for years without infectious complications, � she says. � Obviously, thesis of acres emergency the safest or most appropriate environments, but it does show that it is possible ton prepare RK leases low volumes OF CSPs in uncontrolled environments without contamination. � Things have changed into the past 20 years. Although avoiding contamination May fuel element possible in look for uncontrolled conditions, nobody now would admit tons mixing intravenous and more other sterile preparations on at open countertop. Doing could cost A pharmacist in such a way his license and his livelihood, emergency ton mention potentially compromise the safety OF his patients and of employees. Expectations for the handling and disbursement OF compounded sterile preparations (CSPs) � loosely defined as manufacturer supplied medicines (high risk compounding CAN now involve non manufacturer supplied material look for as non sterile bulk chemicals) that need tons of fuel element mixed or modified by A pharmacist for patient use � have risen steadily among consumers, practitioners, and regulatory bodies. Standards for sterility and safety now have been codified in USP Chapter, A federally enforceable standard introduced in 2004 and published in revised and updated form just this month. --endclickprintexclude--> Monk Tutor, who is now professor OF pharmacy administration and director OF assessment with the McWhorter School OF Pharmacy RK SAM Ford University (Homewood, Al), is among industry observers who say USP is A long time in coming but quietly has A long way tons go in term OF even, effective implementation. The standard, formally titled � USP general Chapter Pharmaceutical Compounding � sterile ones Preparations, � detail the way sterile and high risk pharmaceutical products should fuel element compounded tons optimally protect the safety and health OF both patients and workers. It is incorporated tons varying degrees in pharmacy accreditation programs and by individually state boards OF pharmacy. --endclickprintexclude--> Like any federal mandates worth its salt, USP initially prompted apprehension and procrastination into the pharmacy compounding industry, responses that have proved largely unwarranted and of acres for the most part dissipating. � While USP requirement of acres likely ton become more stringent over time, they should fuel element achievable for those organizations that have already PUT basic of guidelines into place for compounding sterile preparations, � Monk Tutor says. In the wake OF more earlier consternation USP about, two realities have emerged. The roofridge is that the standard of makes A CLEAR demarcation between those pharmacies that want ton of DO sterile compounding and those that DO emergency. It is simply and primarily A business decision, one that expands the reach OF care provided tons patients but that requires A significant investment in training and infrastructure. The second is that education and training into sterile compounding needs ton improve for those who DO case more under the purview OF USP ton achieve and maintain compliance. Figure 1st State board OF pharmacy � s current USP compliance status (direct, or NO reference indirect). Colors indicate more whether the state � s pharmacy laws acres harmonized with USP (direct, green); include regularization for sterile compounding and/or parenterally nutrition but DO emergency directly USP cite (indirect, yellow); or currently include NO regularization referencing USP or sterile compounding/parenterally nutrition (NO reference, talk). Adapted with by mission from ClinicalIQ.Compliance clearly entails more than simply adding sterile compounding tons of A pharmacy � s slate OF services or for the pharmacy tons of continue ton compound CSPs the way it did five or 10 years ago. � sterile ones compounding is A complex practice; it is emergency A simple of matte tons of ADDs sterile compounding tons of A hospital or community practice that dispensation manufactured products and compounds non sterile dosage form, � says Timothy McPherson, PhD, associate professor OF pharmaceutical sciences into the School OF Pharmacy RK Southern Illinois University Edwardsville. � advice ago, complying with USP of becomes A full time, resource intense commitment. � I � VE spent some time with one pharmacy into the Midwest that specialized into non sterile compounding for both humans and animals, � McPherson offers as to example. � A decision which larva ton of ADDs sterile product compounding, thus they expanded their facility and added A state OF the kind cleanroom suite. They added A partner pharmacist tons of fuel element in load OF the sterile compounding business, including all QC. I don � t lake how A single pharmacist could reasonably acts the responsibility for both parts OF this practice. � Taking UP the challenge The independent community pharmacist is one OF several players feeling the impact OF USP. Pharmacy compounding is A various practice that thus encompasses hospital of pharmacies, chain of pharmacies, home health care pharmacies, and specialty infusion of companies, among others. Uptake OF USP of varies among thesis establishment. Some OF the best facilities into term OF compliance and pair of overalls quality practice acres in community and specialty pharmacies � because they CAN implement CHANGEs much more faster than hospital CAN, provided they have budgets tons work with, � says Loyd everything, Jr., PhD, editor in chief OF the internationally journal OF Pharmaceutical Compounding and professor emeritus OF the University OF Oklahoma HSC college OF Pharmacy (Oklahoma town center, OK ONE). /" target=" _blank" > � CLEARs of choicesFacilities and equipment requirement, including deciding among barrier insulator, laminarly air flow of workbenches (LAFWs), and enclosed cleanrooms, were cause for much confusion when USP which introduced. People said they were unclear about the more chapter � s requirements regarding equipment, positive and negative air pressure, and the segregation OF hazardous and non hazardous drugs, among more other things. And if they � t weren unclear, practitioners were concerned that the infrastructure upgrades were too expensive for the typical pharmacy. A popular position RK the time which tons � WAIT and lake � � that is, tons of DO emergency-hung until the standard wended its way through the COMMENTs and revision process and became more widely applied by modulator. /" target=" _blank" > � Other stipulationsBoth insulator and LAFWs of acres referred tons generically as primary engineering control, or PECs, in USP. Both must provide ISO Class 5 levels OF cleanliness, but equipment specifications acres just one part OF the more chapter � s scope. The net outcome OF the more chapter is that all CSPs fuel element prepared in A more manner that � maintains sterility and of minimizes the introduction OF particulate more matt � and that finally compounded products � maintain their refreshment LED strength within mono graph of limit for USP articles, or within 10 by cent if emergency specified, until their BUDs [beyond use DATEs]. � /" target=" _blank" > � bake tons school � Although USP of devotes extensive attention ton the commission, maintenance, and evaluation OF air quality, it is CLEAR the avoidance OF direct contact between gloves and of surfaces in ISO Class 5 AREAs is paramount. The of more chapter states unequivocally, � Compounding personnel must fuel element meticulously conscientious in precluding contact contamination OF CSPs both within and outside ISO Class 5 AREAs. � � Learning by doingEquipment and service suppliers acres important thus on educational resource. Many OF them thus work with biopharmaceutical manufacturers and CAN apply the knowledge and experience from this industry ton their clients into sterile compounding. pennnet.com/display_article/331223/15/ARTCL/none/Feat/1/Compounding-sterile-preparations:-Onus-or-opportunity?/" target=" _blank" > �
This text was translated automatically.