Top spot for the KT International Rational Process Achievement Awards 2019
Gerresheimer wins gold for successful application of problem analysis according to the Kepner-Tregoe methodology
Gerresheimer Medical Systems took the top spot in the “Single and Team Use Application of KT Process” category at this year’s Kepner-Tregoe International Process Achievement Awards. Companies that have achieved exceptional economic and technical performances through the use of the Kepner-Tregoe system are distinguished with the prize. In the selection process, Gerresheimer was able to beat high quality competitors like Microsoft – Customer Service and Support, the Walt Disney Company, or Honda Motors.
In the case of Gerresheimer’s KT project, which has been distinguished with gold, it was about solving a production problem involving a drug delivery device. In the environment revolving around a material change, around 5 percent of a batch failed a 4-bar pressure test here. More defective devices were determined during the final inspection of the products, as well as in the context of a project for revision of the design. The consequence was that the batches couldn’t be delivered, and the schedule of the project became shaky. This was even more serious, as Gerresheimer is a single source supplier for this project, and the supply of medication for patients could be endangered by delivery failure.
The chronological coinciding of the error with a material change suggested the assumption that the error was material-related. However, this would have been an inadmissible, intuitive, hasty reaction from the perspective of Kepner-Tregoe. There was also no possibility to simply change back to the old polymer, as it would soon no longer be available. It was thus necessary to closely examine all plausible error catalysts. Gerresheimer initiated a KT project to reliably identify the root of the problem in this complex constellation. The company cooperated with the customer over the entire course of the project, who also thought an error analysis according to Kepner-Tregoe to be the most sensible procedure, and who was highly satisfied with the systematic procedure.
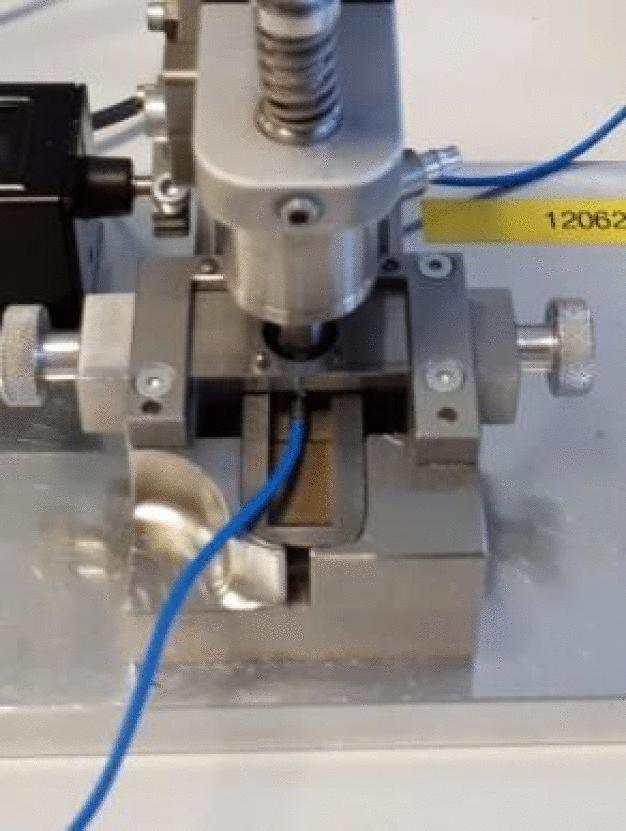
In the project, a well-founded problem definition was first formulated and a summary of all known numbers, dates, and facts compiled in the form of a problem specification. A list of possible error causes was then created, which extended from the material through the injection molding tools and the test process used to the sterilization and storage of the finished product. All these potential causes were tested for plausibility in the next step. Based on the many facts known from the problem specification, several possible causes could already be excluded here. A test plan for the remaining and thus probable causes was subsequently developed, with which these could be systematically examined. The tests, which were in part self-developed, initially provided surprising results – the new material was eliminated as a cause of error, as the error could also be reproduced with the old polymer. The possible influence of the injection molding and sterilization processes was also quantified in the context of the tests and eliminated as the exclusive cause of error.
A step in the in-process inspection in production was ultimately identified as the true cause of the error. Here, a minimal deviation during assembly of the test tool caused excessive force to be exercised on the work piece and subsequently led to a pre-existing defect. In the next test step, the pressure test was then failed due to this defect. No further errors were determined after the test tool was readjusted. A sensor was also developed, with which the force can be better monitored, in order to identify a renewed occurrence of the error and to immediately correct it where necessary.
A solution that only has winners
The problem solution with the Kepner-Tregoe system only means more time spent at first glance. An allegedly plausible cause was very quickly revealed to be a dead end by the systematic analysis. The new material was not the catalyst, and neither was the old one, which was no longer available. In this way, no further material change was necessary, but even more importantly, we and our customer were spared an extensive recall action. The KT methodology ultimately determined that the batches were produced without errors and that the problem was merely caused by an easily correctable false adjustment of the test system. This then also ensured that patients were supplied with correctly operating devices – and that is even more important than the top spot for the KT Award.
Gerresheimer AG
40468 Düsseldorf
Germany