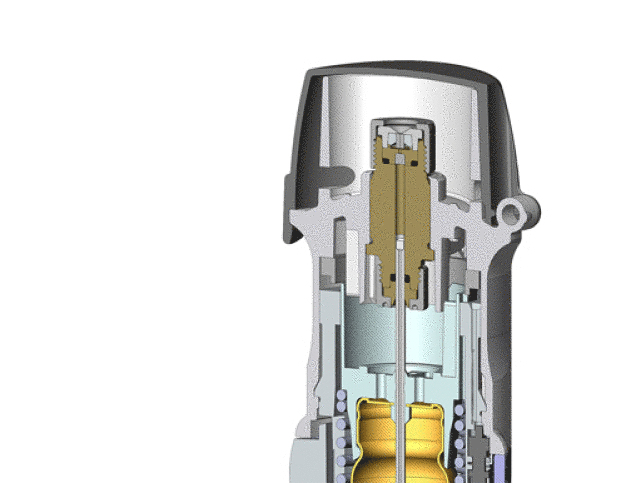
Medical Trends
With today’s aging global population, the medical device industry is poised for a sustained period of rapid growth fueled by a high level of innovation.
Medical device manufacturers and their suppliers face a common challenge: Is it possible to improve financial performance, product innovation and quality while growing at a significant rate?
Tony Christian, Director of Cambashi, an independent industry analyst firm, says it is. In a recent study of the medical device market, Christian and consulting analyst Julie Fraser found the factors necessary to improve profitability within an environment of accelerating product introductions while maintaining high quality standards, despite unavoidable trade-offs.
“Product design, development and introduction are key factors that will improve a life sciences company’s success,” Christian says. “There is always a balance to achieve between innovation and quality. Quality improvement is more daunting with the impact of regulatory and product changes, supply chain complexity and employee skill levels.”
Cambashi’s primary research involving medical device and life sciences companies and their suppliers focuses on strategies to grow and simultaneously increase business performance in areas such as cost containment, earnings, net operating profit and return on assets. “In fact, the best improvement opportunities relate to product innovation – that is, expanding the product set by rolling out new products and product lines or adding to existing product lines,” Christian says.
Medical device manufacturers and their suppliers must become more agile to better manage the balance between quality, compliance and innovation, the study found. Material suppliers to the life sciences industry have quickly come to understand this and are catering even more to the needs of their customers. The combination of new products and higher volume signifies increased revenues. However, it also brings unprecedented challenges for the regulatory, production and supply chain departments. In some cases, growth can actually hurt profits.
“Product changes, whether engineering, technology or material changes, pose one of the most significant challenges, especially with quality,” Christian says. “As product specifications change, so do quality targets, testing procedures and an array of processes. It’s difficult to get beyond a baseline and document the processes in the face of constant change. Product change ripples through every aspect of the organization, not just quality and regulatory compliance.” Supply chain issues rank high on the list of challenges, as product and regulatory changes impact suppliers. The U.S. Food and Drug Administration and other regulators make it clear that device makers are responsible for the quality and compliance of their suppliers. While suppliers have caused various problems in meeting standards, they rarely cause audit findings or compliance issues. Christian explains that medical device manufacturers must also be very careful in choosing their suppliers in light of the fact that they could cause problems in quality, obsolescence, manufacturing, costs and delays.
“Unfortunately, most companies do not have full visibility into materials compliance issues and may incur extra costs if they don’t catch environmental issues early in design,” he says.
More mature manufacturing industries that make products whose quality can impact human safety – such as automotive and aerospace – are more likely to use supplier quality processes. Aerospace companies commonly have supplier quality personnel who go on site with suppliers for improvement projects.
“Bringing better practices to lower costs and improve quality is a way of life for those industries,” Christian says. “Of course, that is challenging to do unless the company has its own quality processes that are well established and run effectively. Other industries are also working to master materials environmental compliance. Consider whether you can find suppliers that also sell into those industries, as strong capabilities can be a positive selling factor for them.”
Product innovation is essential to success in the life sciences industry. It often appears as a wide range of product families, and in products with many variants or configurations. Therefore, says Christian, it is important to select the right suppliers that can support new product initiatives. “Innovation is highly dynamic as new medical devices are developed, technologies improved, regulations changed and treatments adopted worldwide,” he says. He counsels manufacturers to ask themselves: “Have you invested the time needed to adopt better practices and determine how to choose suppliers that will give you a competitive edge?”
Trelleborg in Life Sciences
Trelleborg Sealing Solutions develops, manufactures and supplies innovative engineered solutions for demanding medical, biotech and pharmaceutical applications in thermoplastics, silicone and other elastomers. Sandro Silverio, Global Director at Trelleborg Sealing Solutions with responsibility for life sciences, says: “We are an established and respected supplier to the life sciences industry. As Tony Christian points out, it has been our processes that have made us successful. As we supply so many industries and are a major supplier of sealing systems to automotive and aerospace manufacturers, the stringent quality disciplines required in these sectors have been seamlessly transferred to our life sciences customers.”
Trelleborg Sealing Solutions Silcotech AG
8260 Stein am Rhein
Switzerland