Paul Lopolito, Elizabeth Rivera, STERIS Corporation, Life Science Division
Addressing Microbial Contamination in Process Equipment
Introduction:
Microbial contamination is recognized as a source of risk to the consumer. The presence of objectionable microorganisms in non-sterile products, or any type of microorganism in sterile products, denotes inadequate process controls. The control of microbial contaminants requires the interaction of a multidisciplinary group to identify root causes and to implement corrective actions.
Purpose:
This article assembles information to assist in this investigational process and offers a holistic approach to remediate microbial contamination in process equipment.
Part I: Pre-inspection work:
The pre-inspection review should include: identification of the microorganism (genus and species); the history of microorganism onsite; process, personnel and waste flow diagrams; equipment drawings; and equipment cleaning procedures and sanitization practices. Understanding this information allows an auditor to investigate intrinsic or extrinsic sources. Intrinsic sources are those integral to the manufacturing process or equipment. Extrinsic sources are those associated with a transfer into the product via air, liquid or surface contact. Historical information regarding prior microbial contamination can be helpful in understanding whether the contamination is from a single incident, or from insufficient practices or controls.
Microbial contamination can be introduced from a number of sources (figure 1) including Gram-negative and Gram-positive bacteria, mycobacteria, mycoplasma, viruses and fungi (mold and yeast). Bacterial spores are a concern due to their high resistance to destruction. Gram-negative bacteria are often associated with biofilm in process systems or utilities. The presence of biofilm often involves a modification of the cleaning procedure to effectively remove the residue.
Part II: Inspection:
The inspection process should consider potential causes of microbial contamination and focus on areas of high risk.
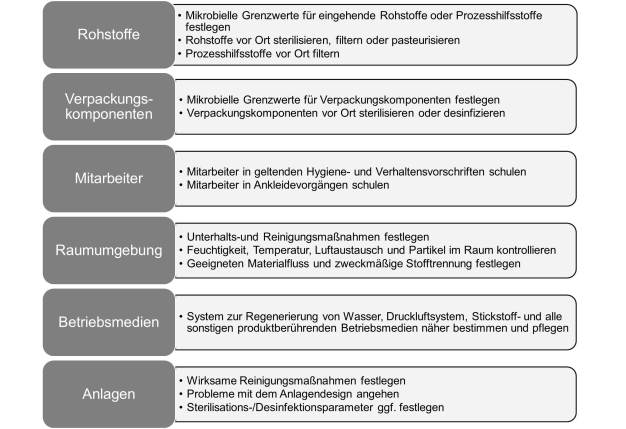
Raw materials, processing aids and packaging
A microbial risk assessment of each raw material from each supplier should be performed. Microbial reduction of the raw material through pasteurization, filtration or sterilization can be performed before use if warranted. All recent changes in raw materials or product formulations should be investigated. Storage, sampling and dispensing of raw materials should be reviewed for possible ingress of microorganism. Any processing aids or packaging components used during the manufacturing process should also be assessed for microbial risk.
Personnel and room environment controls
Personnel are the largest contributor of viable and non-viable particles. The way to control viable and non viable particulate contamination via personnel is to control access and attire, and to provide rigorous training in gowning procedures and appropriate clean room behavior. Other environmental controls include process equipment selection, and cleaning and disinfection practices (Bartnett, 2007 and USP 1072).
Utilities (water, steam, compressed air and gases)
Any utilities used in direct contact with the product should be inspected, and routine monitoring test results and contamination control practices reviewed. This might include routine microbial, total organic carbon (TOC) and endotoxin testing. Filters and ultraviolet (UV) bulbs should be changed per manufacturer recommendations. Common bioburden reduction steps for water systems include distillation, filtration and/or UV treatment. Compressed air and gases should be filtered before use to prevent viable and non-viable particles, oil, and condensation from entering the process system. Non product contact utilities, such as cooling or heating systems, should be inspected for possible cross-contamination with product or product contact surfaces.
Walk-through of process, personnel and waste flow
It is important to walk through the manufacturing process and observe how each raw material, utility, and processing aid is introduced or exposed to the product. The auditor can assess possible intrinsic and extrinsic risk and verify controls. Personnel from quality, engineering and operations should accompany the auditor to address questions. Photographs and additional sampling data outside of routine environmental and product samples are helpful when seeking support for changes to contamination control practices.
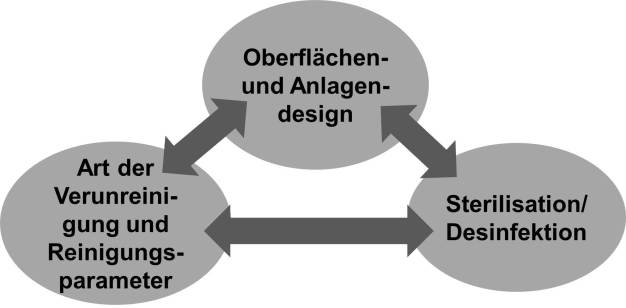
Processing equipment and microbial decontamination
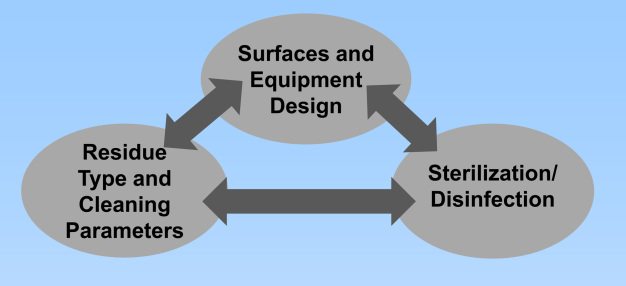
The major elements that must be considered when addressing microbial contamination in processing equipment are the surfaces and equipment design, residue type and cleaning parameters, and sterilization / disinfection. Deficiencies found in one element may have a significant impact on the others, and vice versa. (see figure 2)
Residue type and cleaning parameters
A successful cleaning process is necessary for microbial control. Indeed, many biocidal agents require a clean, soil-free surface before application or their microbial efficacy is reduced.
Process changes in manufacturing steps, batch composition, dirty hold time, raw material sources, environmental factors and other elements may affect the soil condition at the time of cleaning. If changes have occurred, then lab evaluations are recommended to confirm the adequacy of the current cleaning process. This is done by testing the four key parameters that govern cleaning effectiveness. These parameters are the contact time of the cleaning solution with the dirty surface; the action or force acting on the surface; the concentration of the cleaning agent; and the temperature of cleaning solution [Verghese, 2009]. In addition, if the residue was dried, baked, autoclaved, denatured, or polymerized onto the surface, it is important to mimic the actual conditions at time of cleaning, including the amount of soil present on the surface, the tenacity of the soil adhesion, and the extent of dispersion of the soil in the cleaning solution during the cleaning process.
Laboratory evaluations should also be used during a microbial contamination investigation. A lab study helps to identify any difficulties with the efficacy of soil removal and to access the impact of residual soil on microbial contamination risk in the system. (see figure 3)
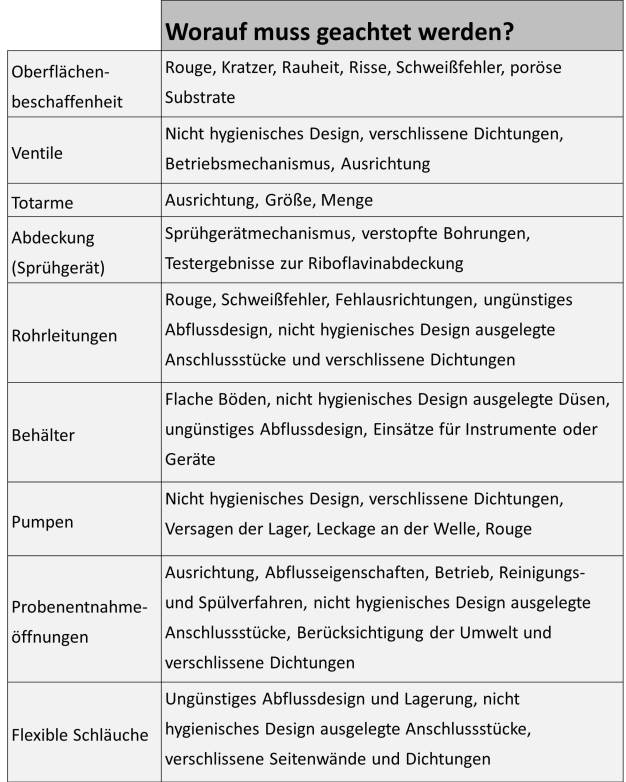
Surface and process equipment design
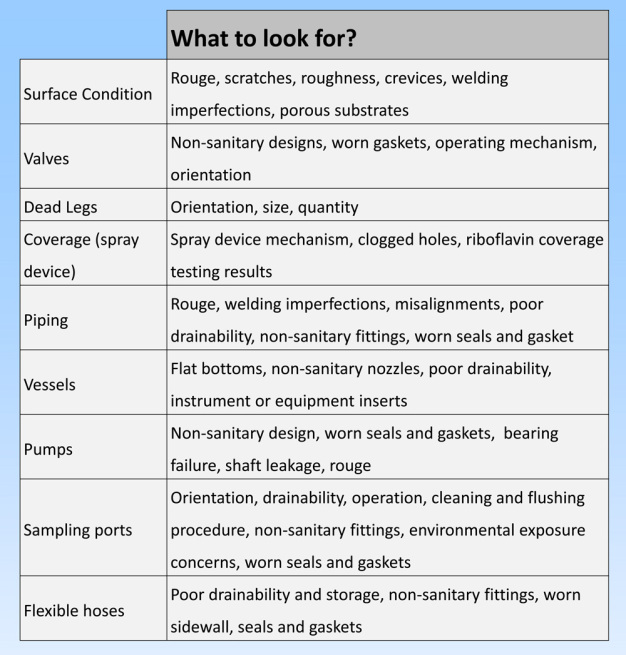
Surface characteristics and equipment design can affect successful cleaning and microbial efficacy. Surface characteristics such as rouge and scale may be removed chemically, however scratches, crevices and corrosion will need more aggressive maintenance. Process residues and microbes might adhere more tenaciously to the irregular surface and become more difficult to remove. Equipment design elements such as coverage, flow rate, component selection, size and orientation of dead legs, valve selection, drainability and pitched piping are also important factors for successful cleaning and sanitization (Rivera, 2012 and Verghese, 2009). (see figure 4)
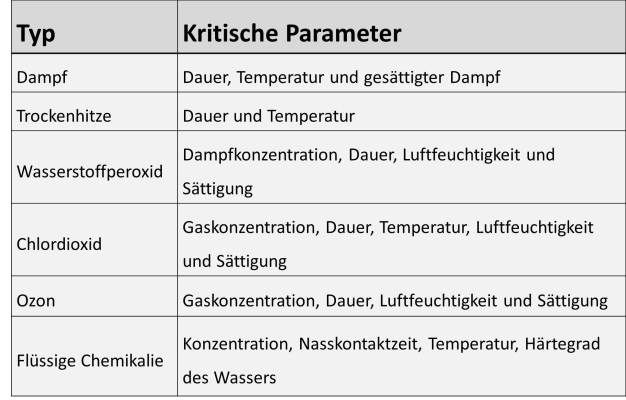
Sterilization or disinfection parameters
Several technologies are available for disinfection or sterilization of equipment. High temperature sterilization techniques would be steam-in-place and dry heat. Gas/Vapor technologies include vaporized hydrogen peroxide, chlorine dioxide, and ozone technologies. Chemical sterilization can also be performed using commercially available sporicidal/sterilant agents.
In the case of non-sterile pharmaceuticals, it is neither required nor expected to sterilize processing equipment. Generally, a low and controlled bioburden is acceptable. An effective cleaning procedure followed by a disinfection or sanitization step may be sufficient, provided these can kill objectionable microorganisms. References such as the USP “Microbiological Attributes of Non-sterile Products” provide assistance in defining and setting microbial limits for finished products. Nonetheless, the significance of other microorganisms must be established on a case-by-case basis. These are microbial contaminants that, depending upon species, cell number, dosage form and intended use of the drug, may adversely affect patient safety [USP 1111]. The aforementioned methods should be validated to ensure that they will consistently meet pre-established criteria. Change in the soil level, microbial load or species, and surface attributes can impact the disinfection or sterilization method being used. Critical parameters governing the effectiveness of any of these methods must be periodically monitored to verify the validated state. (see figure 5)
Conclusions:
Contamination of a product with an objectionable microorganism is a costly and time consuming problem that needs to be addressed quickly and efficiently by a cross-functional team. It is critical to identify the microorganisms early in the investigation and determine whether they are from an intrinsic (of the manufacturing process) or extrinsic (outside of the manufacturing process) source. A careful walk-through of the process can be used to flag areas of microbial risk; identify the presence or lack of controls; understand when the product is at highest risk; and provide solutions to reduce the risk. A holistic approach is commonly used to address contamination issues. As part of a holistic approach, assess the cleanability of the system and make engineering changes to address issues such as coverage, drainability, etc. as needed. Perform a robust high temperature, alkaline cleaning to remove process and microbial residue. If rouge or water scale is present, perform a high temperature acid cleaning to remove the inorganic residue. When possible, use a disinfection/ sterilization procedure that is routinely performed onsite, and ensure critical parameters are achieved during the process. Once the manufacturing process is back up and running, schedule regular meetings to review current microbial control measures and internal plans to quickly and effectively address the next microbial contamination event.
References:
- Bartnett, C., Polarine, J. and Lopolito, P. “Control Strategies for Fungal Contamination in Cleanrooms”. Controlled Environments, Sept. 2007.
- Rivera. E. “Basic Equipment Design Concepts to Enable Cleaning in Place”. Pharmaceutical Technology. http://pharmtech.findpharma.com/pharmtech/article/articleDetail.jsp?id=726190.
- Verghese, G. and Kaiser, N. (2009). Cleaning Agents and Cleaning Chemistry. Pluta, P (eds) Cleaning and Cleaning Validation Volume I, Davis Healthcare International and Parenteral Drug Association (2009), Chapter 7, Pages 103-121.
- Verghese, G. and Lopolito, P. (2009). Cleaning Engineering and Equipment Design. Pluta, P (eds) Cleaning and Cleaning Validation Volume I, Davis Healthcare International and Parenteral Drug Association (2009), Chapter 8, Pages 123-150.
STERIS Deutschland GmbH
50933 Köln
Germany