Carol A. Bartnett, Jim Polarine, Tom Smith, Dan Klein, Peter Karanja
Fungicidal activity of globally acceptable quaternary ammonium disinfectants
ABSTRACT
Quaternary ammonium compounds (QACs) are excellent active ingredients in disinfectant products because they have low toxicity, good detergency, and bactericidal efficacy. Unfortunately, many currently marketed QAC products are incompatible with some forms of sterilization and provide insufficient fungicidal activity. These drawbacks can prevent the use of QAC disinfectants in ISO-5 cleanrooms. In addition, some QACs have limited regulatory acceptability in certain European countries. The studies described here demonstrate that a product formulated with didecyl dimethyl ammonium chloride, a QAC, has activity against multiple fungal strains, including Aspergillus brasiliensis, is irradiation-stable, and meets global standards for environmental acceptability.
INTRODUCTION
Quaternary ammonium compounds have been used as active ingredients in hard surface disinfectant products since the 1930’s, with hundreds of iterations now available. The structure of these compounds includes a positively charged nitrogen atom with four organic groups attached (1). Over the years, various QACs have been developed with different combinations of alkyl and aromatic groups bonded to the nitrogen atom, and these formulations are now used globally (2).
An important factor affecting the selection of a QAC for a disinfectant formulation is whether it is accepted by appropriate regulatory bodies including the United States Environmental Protection Agency (EPA) and the European Biocidal Products Directive (BPD). The number of QACs that are EPA-registered and actively supported for the BPD is relatively small, only including such QAC active ingredients as didecyl dimethyl ammonium chloride and alkyl dimethyl benzyl ammonium chloride (3,4). This can make the selection of a quaternary ammonium disinfectant challenging for international pharmaceutical companies interested in harmonizing global practices. This challenge becomes even greater when selecting a disinfectant for use in a controlled environment.
Disinfectant products must be sterilized before introduction into the cleanroom (5). One method to achieve sterilization of the product and packaging is through exposure to gamma irradiation. QACs with alkyl groups have demonstrated better stability to gamma irradiation than QACs with aromatic groups. When an aromatic QAC is exposed to gamma irradiation, the bond between the nitrogen atom and the aromatic portion of the molecule can break, resulting in the formation of amines as by-products. Table I compares the stability to gamma irradiation of an aromatic QAC formulation to that of an alkyl QAC formulation. Degradation of the alkyl QAC from exposure to gamma irradiation is minimal, whereas degradation of the aromatic QAC is significant and increases as the irradiation dose increases (6). (See table I)
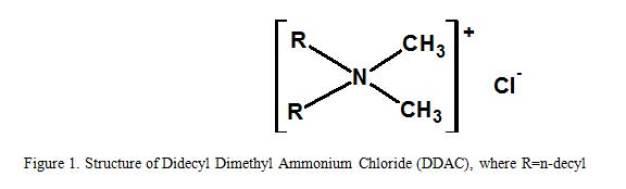
When combining the regulatory criteria with the desired irradiation stability, the best remaining choice among QACs for a global disinfectant formulation is didecyl dimethyl ammonium chloride. (See figure 1)
Although didecyl dimethyl ammonium chloride meets requirements for international use, some QAC formulations lack true broad-spectrum activity. While demonstrating bactericidal and virucidal activity, some QAC products lack the required efficacy against certain types of fungi due to various mechanisms of intrinsic resistance and the formation of fungal spores that are more resistant to disinfection (7). The consequences of fungal contamination can be significant and lead to long-term problems within a facility. FDA (Food and Drug Administration) warning letters and Form 483 observations often address inadequate control of fungal contamination and lack of assurance that disinfectants used in a facility are effective against fungi (8,9).
One particular fungal strain, Aspergillus brasiliensis 16404 (formerly known as Aspergillus niger 16404), has proven to be a difficult challenge for pharmaceutical companies, and is not addressed by most QAC-based disinfectants (10). In many cases, sporicides and disinfectants such as bleach (sodium hypochlorite), hydrogen peroxide, peracetic acid, chlorine dioxide, ozone, glutaraldehyde, iodine, and phenols are used in applications that require efficacy against Aspergillus brasiliensis. However, use of some of these compounds can have safety, environmental, odor, or staining issues. A QAC-based disinfectant product with recognized Aspergillus brasiliensis claims gives users a safer alternative for this type of application.
When formulating a QAC-based disinfectant, ingredients such as an alkalinity source, chelant, co-solvent, or surfactant can be selected to enhance the activity of a formulation based on didecyl dimethyl ammonium chloride. These ingredients are also compliant with the REACh (Registration, Evaluation, Authorization, and Restriction of Chemical substances) regulation (11). Compliance with this regulation is expected to eventually become mandatory for all formulations sold in the European Union.
The testing described in this article was performed on a formulation based on didecyl dimethyl ammonium chloride against various fungal strains using European and United States EPA-approved methodologies.
MATERIALS AND METHODS
Efficacy testing for United States and European disinfectant label claims requires strict adherence to relevant standardized methods recognized by the appropriate regulatory authorities. The procedures are summarized below.
BSEN 1650 Method (12)
Test organism preparation. Suspensions of Candida albicans ATCC 10231 and Aspergillus brasiliensis ATCC 16404 were prepared and adjusted to achieve approximately 1.5 – 5.0 x 107 cfu/mL. Serial ten-fold dilutions were performed to verify the number of CFU (colony forming units) per mL in the inoculum suspension.
Test Procedure. An aliquot of the disinfectant diluted in 300 ppm calcium carbonate (CaCO3) hard water was added to a tube containing the interfering substance and fungal suspension and held at 20 ± 1°C for the specified contact time. At the end of the contact time, 1.0 mL the test mixture was transferred to neutralization broth. After a neutralization time of 5 minutes ± 10 seconds, a 1.0 mL sample of each neutralized mixture was added to a sterile petri dish. Molten Malt Extract Agar (MEA) was added to each petri dish. Following incubation, all plates were counted and the CFU/mL of the test mixture was calculated. Validation testing and appropriate controls were performed concurrently with the test procedure as outlined in BS EN 1650.
BS EN 13697 Method (13)
Test organism preparation. Suspensions of Candida albicans ATCC 10231 and Aspergillus brasiliensis ATCC 16404 were prepared and adjusted to achieve approximately 1.5 – 5.0 x 107 cfu/mL. Serial ten-fold dilutions were performed to verify the number of CFU (colony forming units) per mL in the test suspension. The test inoculum was prepared by adding 1 mL of each fungal suspension to 1 mL of the interfering substance (3.0 grams BSA in 1 liter of distilled water) and mixed well.
Test procedure. Clean, dry stainless steel discs two centimeters in diameter were placed into shallow individual sterile containers. The test surface was inoculated with 0.05 mL of the prepared test inoculum and dried at 37°C until visibly dry. An aliquot of the disinfectant solution was placed on each test surface ensuring that the dried inoculum was completely covered. After the specified exposure time (5 min for C. albicans and 15 min for A. brasiliensis), 10 mL of neutralization broth was added. Each container was covered and mixed for 1 minute to remove any remaining cells/spores from the surfaces. After a neutralization time of 5 min ± 10 sec, the neutralized mixture was serially diluted and a 1.0 mL sample of each dilution in duplicate was added to separate sterile petri plates followed by molten MEA agar. The test surface was recovered, rinsed with 10 mL of sterile distilled water and, with the test side facing up, transferred to a petri dish containing about 10 mL of solidified MEA. An aliquot of sterile distilled water was placed on the disc and the surface was scraped with a sterile spatula for 1 minute to remove residual desiccated inoculum on the disc surface. An additional 10 mL of molten MEA agar was poured over the disc. Following incubation, all plates were counted and the number of colony forming units was recorded. Validation testing and controls were performed concurrently with the test procedure as specified in BS EN 13697:2001.
AOAC Fungicidal Methodology (14)
Test organism preparation. Fungal cultures of Aspergillus brasiliensis ATCC 16404, Aspergillus niger ATCC 6275 and Trichophyton mentagrophytes ATCC 9533 were propagated in Neopeptone Glucose Agar (NGA) at 25-30°C for 7 to 10 days. The mycelial mats were dislodged from the agar surface and macerated with saline solution in a sterile glass tissue grinder. This was followed by filtration through sterile glass wool. The final inocula were prepared by adding the appropriate amount of Fetal Bovine Serum (FBS) to each culture to achieve a 5% mixture. The density of each conidial suspension was determined using the plate count method. Prior to use, the respective suspensions were standardized using saline solution to yield approximately 5.0 x 106 conidia/mL.
Test procedure. For each test organism, two 25 x 150 mm test tubes containing 5 mL representing each lot of the test substances were equilibrated to 20±2°C. A volume of 0.5 mL of standardized filamentous fungal inoculum was added to each tube and swirled. After 10 minutes contact time at 20±2°C, a sample from the tube was transferred into 10 mL of appropriate neutralizer using a 4-mm microbiological loop. The process was repeated for all tubes. Appropriate controls were also performed as outlined in the AOAC Official Method 955.17 Fungicidal Activity of Disinfectants. The tubes were thoroughly shaken following each transfer and all tubes were incubated for 7 to 10 days at 25-30°C. After incubation, the tubes were observed for the presence or absence of growth.
Time Kill Method
Test organism preparation. A fungal culture of Aspergillus brasiliensis ATCC 16404 was grown on a Sabouraud Dextrose Agar (SDA) slant for 7-10 days at 25-30°C. The working microbial inoculum was prepared by dislodging the mycelial mats from the agar surface and macerating with saline solution in a sterile glass tissue grinder.
Test procedure. A volume of 0.1mL of the working microbial inoculum was transferred into 9.9 mL of disinfectant. After 1, 5 and 10 minutes contact time, a 0.1mL sample from the tube was transferred into 10 mL of neutralization broth. Ten-fold serial dilutions were then performed, plated and poured with SDA. Plates were incubated at 30°C for 5-7 days. Controls were performed in the same manner but using buffer in lieu of the disinfectant. Log10 values of CFU/mL data were calculated for controls and test articles. Log10 reduction values represent the difference between log10 average control values and the log10 of test article values.
RESULTS AND DISCUSSION
BS EN-1650 is a quantitative suspension test used to evaluate fungicidal activity of disinfectants. Since the test conditions are representative of practical use, this method can be used for generic disinfectant claims in many European countries. The pass criterion for the test is ≥ 4 log10 reduction of viable counts (Log R). Table II shows that the test product diluted to 1:128 in hard water and tested at 20° ± 1°C under dirty conditions achieved >4.5 Log R to demonstrate fungicidal activity against Candida albicans ATCC 10231 at 5 minutes. Table III shows that the test product diluted to 1:32 in hard water and tested at 20° ± 1°C under dirty conditions achieved >4.6 Log R to demonstrate fungicidal activity against Aspergillus brasiliensis ATCC 16404 at 15 minutes. (See tables II and III)
BS EN 13697 is a quantitative surface test to establish that products have microbicidal activity against surface-attached microorganisms. The pass criterion for fungicidal efficacy is ≥ 3 log10 reduction, which is calculated as the microbicidal effect (ME value). Table IV shows that the test product diluted to 1:128 in hard water and tested at 20° ± 1°C under dirty conditions (3 g/L BSA) demonstrates fungicidal activity against Candida albicans ATCC 10231 at 15 minutes with a ME value of >5.66.
Table V shows that the test product diluted to 1:32 in hard water and tested at 20° ± 1°C under dirty conditions (3 g/L BSA) demonstrates fungicidal activity against Aspergillus brasiliensis ATCC 16404 at 15 minutes with a ME value of >5.66. (See tables IV and V)
In the United States, fungicidal efficacy is determined using AOAC Official Method 955.17 Fungicidal Activity of Disinfectants. Results showed that the test product was effective against Trichophyton mentagrophytes ATCC 9533 and Aspergillus niger ATCC 6275 after a 10 minute exposure period when diluted 1:128 in 400 ppm hard water and tested in the presence of a 5% fetal bovine serum organic load using AOAC fungicidal activity test with no growth observed in any of the test replicates. The test product also showed efficacy against Aspergillus brasiliensis ATCC 16404 when diluted 1:64 in 400 ppm hard water and tested in the presence of a 5% fetal bovine serum organic load after 10 minute exposure period. Experiments have shown that Trichophyton mentagrophytes was more susceptible to the disinfectant than Aspergillus brasiliensis ATCC 16404 (15).
Basic activity of products containing QACs against Aspergillus brasiliensis ATCC16404 can be determined using a time kill suspension test as shown in Figure II. Products A and B are household ready-to-use QAC cleaner/disinfectants and products C, D, E, F are pharmaceutical grade QAC disinfectants. Product C also contains a biguanide.
The results in Figure II demonstrate the importance of proper formulation in the development of QAC disinfectants. A significant difference can be observed in effectiveness against Aspergillus brasiliensis that does not directly correlate with active ingredient type, concentration or market application. (See figure II)
CONCLUSION
The use of a quaternary ammonium compound disinfectant has multiple benefits including excellent cleaning detergency, low toxicity, and bactericidal and virucidal efficacy. However, not all quaternary ammonium compound formulations offer the fungicidal activity of other active ingredients, and marketed QACs can demonstrate widely varied levels of activity against fungal spores. However, a properly formulated QAC-based disinfectant can demonstrate activity against challenging fungal species such as Aspergillus brasiliensis without sacrificing other key attributes such as globally acceptable ingredients, environmental acceptability and irradiation stability in a concentrated form.
REFERENCES
1. M. Cucci. Soap and Sanitary Chemicals. 25, 129-134,145 (1949).
2. P. Schaeufele. J. Assoc. Ocs. 61, 387-389 (1984).
3. U. S. Environmental Protection Agency, Substance Registry Services, http://iaspub.epa.gov/sor_internet/registry/substreg/searchandretrieve/substancesearch/search.do , accessed July 13, 2011.
4. Regulation (EC) No. 2032/2003. List of participants/applicants to the Review Programme of existing active substances used in biocidal products, http://ec.europa.eu/environment/biocides/pdf/list_participants_applicants_subs.pdf , accessed July 13, 2011.
5. FDA Guidance for Industry, Sterile Drug Products Produced by Aseptic Processing – Current Good Manufacturing Practice, U.S. Department of Health and Human Services, Food and Drug Administration, September 2004.
6. STERIS Corporation internal data, unpublished.
7. McDonnell, G.E. “Antisepsis, Disinfection, and Sterilization: Types, Action, and Resistance, ASM Press, Washington, DC, 2007.
8. U.S. Food and Drug Administration, Inspections, Compliance, Enforcement, and Criminal Investigations, Medimmune, Inc. Warning Letter, May 27, 2007, http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/2007/ucm076398.htm , accessed May 9, 2011.
9. GMP Trends Inc., Issue 766, December 15, 2008.
10. Polarine, J., Macauley, J., Karanja, P., Klein, D., Martin, A., “Evaluating the Activity of Disinfectants Against Fungi,” Cleanrooms: The Magazine of Contamination Control Technology, 23 (2), February 2009.
11. Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACh), establishing a European Chemicals Agency, amending Directive 1999/45/#C and repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC, http://eurlex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:32006R1907:EN:NOT , accessed July 11, 2011.
12. BS EN 1650:2008. Chemical disinfectants and antiseptics – Quantitative suspension test for the evaluation of fungicidal activity of chemical disinfectants and antiseptics used in food, industrial, domestic and institutional requirements (phase 2, step 1). Available from British Standards Institute (BSI), 389 Chiswick High Rd., London W4 4AL, U.K., http://www.bsi-global.com .
13. BS EN 13697:2001. Chemical disinfectants and antiseptics – Quantitative non-porous surface test for the evaluation of bactericidal and/or fungicidal activity of chemical disinfectants used in food, industrial, domestic, and institutional areas – Test method and requirements without mechanical action (phase 2/step 2). Available from British Standards Institute (BSI), 389 Chiswick High Rd., London W4 4AL, U.K., http://www.bsi-global.com .
14. AOAC Official Method 955.17 “Fungicidal Activity of Disinfectants,” Official Methods of Analysis of the AOAC, Eighteenth Edition, AOAC International, 2005.
15. STERIS Corporation internal data, unpublished.
STERIS Deutschland GmbH
50933 Köln
Deutschland