High-purity water analytics � as are cleanly your high-purity water?
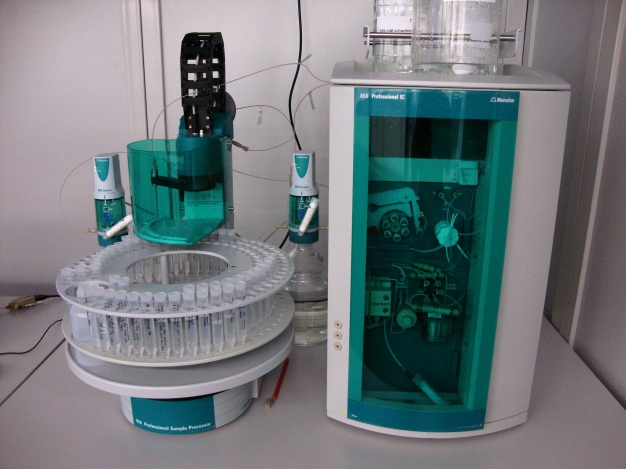
Analytics of pure and high-purity water are a very comprehensive topic with very different requirements and limit values in the appropriate branches of industry. In atomic power plants among other things the borrowing stop is constantly supervised by high-purity water. It is valid to surely prove metallic contamination in high-purity water for the semiconductor industry within the lower ppt range. Pharmawasser is particularly examined for micro-biological contamination. For example if chloride within the ppt range must be proven in trace analytics, it is valid to keep the ubiquitously occurring chloride in the Eluenten so small as possible. That extensively well-known parameters of the specific conductivity of high-purity water of 18,2 M�/cm can represent therefore only a first reference point in the view of the quality of the examined high-purity water. In this short overview are exemplarily and extensively admitted some analytic checking methods for the investigation of high-purity water to be outlined. Depending upon question there are beyond that numerous methods, which are purposefully at the respective range of application cut. In molecular biology for example it is valid to make high-purity water available without impurities with DNA or RNA fragments. This parameter is proven for example with PCR analytics. The following investigations accomplished by the Fraunhofer IPA become among other things for those� Purely (st) water analytics applied: � micro-biological contamination (bacteria, mushrooms, spores) � total carbon (inorganic and organic) � conductivity as sum parameters of all ionischen connections � ion chromatography for the quantitative determination of individual ions � gas chromatography for quantitative regulation more organically pollution on the determination of total carbon is not entered more in greater detail in this article. This effected in one the next expenditure conductivity by a conductivity measuring instrument is measured thereby the specific conductivity of the high-purity water. This represents a very robust and economical method, which in high-purity water systems on-line usually according to standard is used. The measured specific total conductivity adds itself from the specific single conductivenesses of all existing ions (ion equivalent conductivity �; in [S � cm2 � mol � 1] or [� � 1 � cm2 � mol � 1]). On the basis of the respective molecular ionic conductivities thus the specific conductivity of a salt solution can be computed. Importantly�� Conductivity measurements is the temperature, which exhibits the solution which can be measured during the measurement. With most measuring instruments this is seized with and considered accordingly. The expenditure takes place then on a reference temperature, for example T = 25 �C. Admit are the following units: the resistivity in �cm and the specific conductivity in S � cm-1. Usually the measured value is indicated as resistivity, whereby is valid: 1 S � cm-1 = 1 � � 1cm-1. In the following table exemplarily the most important ion equivalent conductivenesses is represented (3). Kaliumchlorid (KCl) possesses a molecular ionic conductivity with 25 �C of 74 + 76 = 150 cm2 � �-1 � mol-1. 1 mm a solution of Kaliumchlorid (about 75 mg/l) corresponds therefore points with 25 �C a conductivity of 1,5E-4 cm-1 � �-1 or 150 �S/cm up. In practice the value with 147 is easily smaller �S/cm due to the fact that the ion equivalent conductivity is based on a theoretical infinite dilution (Kohlrausch law (4) or Debye H�ckel Onsager theory (5)). Limit values for the conductivity are for the different high-purity water qualities among other things in the Ph.Eur (1), in the standard ISO 3696 (6) in the IRTS Roadmap (7) and ASTM D1193-06 (8) defined. Important it is to be noted with the fact that water exhibits a small intrinsic conductivity also in the absolutely pure condition due to its characteristic as Ampholyt. This lies with approximately 18.2 M�cm or 0.55 �S/cm with 25 �C. If the analysis of the conductivity does not ion-chromatography-hand no more as sum parameter of all ionisch available connections in the high-purity water which can be analyzed due to detail depth lacking out, the ion chromatography with enrichment comes to the measurement of ions in the ppt range to the employment. Here the high-purity water which can be analyzed is given with the help of an automated sample giver on an enrichment column. All ionischen connections (either cations or anions, depending on which enrichment and analysis column is inserted and is driven which method) are held back thereby by the enrichment column. Thus a much larger sample volume can be analyzed due to its compared with conventional sample loops that by the enrichment and following Elution despite large analyzing volumes again a very sharp peak can be achieved. In the actual chromatography the ions in the analysis column are isolated temporally depending upon mobility and analyzed afterwards usually by means of a highly sensitive conductivity detector. For special questions further partly selective detection technologies, like among other things UV/VIS detection, exist amperometrische detection and coupling techniques for example with a mass spectrometer (9). On machine details like the used chemical Suppression with anion analytics is � here not to be received further. Based on a calibration and the ion-specific retention time (retention time in the analysis column) the concentration of the respective ion can be determined by a surface comparison of the individual curves. Exemplary the influence of the advance before the actual withdrawal from a point of usage for the institute-own high-purity water ring plant is pointed out with the help of an anion chromatography with enrichment and conductivity detection with chemical Supression. As machine equipment for anion analytics within the trace range a system based from that 850 Professional IC as well as that 858 Professional SAM-polarize Processor, two 800 Dosino and the software MagIC Net (Metrohm AG, Herisau, Switzerland) used (illustration 1).Es was selected an enrichment of the anions with conductivity detection with chemical Suppression. The calibration curves were automated provided with the help of the sample giver on the basis of a master solution and different enrichment volumes. Thus a very high accuracy under exclusion of possible back contamination could be achieved by outside and interiorlateral rinsing the sample giver aristocracy after each injection. The following results could be determined: It could be shown that, although on-line measurement pointed the demanded value of 18,2 M� out with the high-purity water plant at an exemplary point of usage up to a lead time by 5 minutes different anions could be proven. At this point of usage a lead time is recommended by ten minutes, in order to get perfect high-purity water. To the illustration: 1 �g/l chloride after five minutes advance from a 1 �g/l potassium chloride solution (0.013 �mol/l KCl correspond) result in for example a theoretical conductivity of 0,0024 �S/cm (see table 1). Further the influence of the purity of the used sample containers plays a considerable role and must in blank determinations with be always analyzed. Ioni impurities on technical construction units (here exemplary 4 mm a socket-head cap screw; 10 mm long) can be quantified with the ion chromatography outstanding. Generally glass containers for trace analytics should not be used due to leaching out ions by the very aggressive high-purity water. Suitably are among other things containers from highly pure polypropylene (PP). All PP-containers (production of the Eluenten, sample handling and - storage) are constantly stored in high-purity water, which in defined time intervals exchanged wird.Gaschromatographie/MassenspektroskopieDie know molecules polluting the high-purity water by different procedures on the column of a gas chromatograph (office) are given up. Pending of the characteristic of the molecules (size of the molecule, reciprocal effect with the stationary matrix of the column among other things) these walk with different speed by the column and arrive at different times at the detector. As detector if a mass spectrometer (ms) is used, in this the molecule which can be analyzed is divided through for example electron bombardment into fragments (fragmented). Depending upon relationship mass to the load molecule fragments (mean simply loaded) these are detected. Thus one receives a certain spectrum of the ms at each time of the Chromatogramms from that office. The following task procedures can be used: Liquidinjection with this method a certain volume of the high-purity water which can be analyzed is injected into a gas chromatograph (office) and given in its injector verdampft.HeadspaceDabei the high-purity water into a cleaned office glass container (Headspace Gl�) and locked with a septum. It stops itself then for each component an equilibrium between liquid and gaseous phase. The equilibrium can be shifted by keeping at a moderate temperature strongly toward gaseous phase. From the gas area then a part is inferred constantly rinsed from the injector office supplied. of the Headspace TrapAbweichend by means of gas densities a syringe and to Headspace analytics thereby Gl� with Reinstgas (helium or nitrogen etc.). The Sp�lgas will be held back afterwards over cooling case led, on which all organic compounds of a certain, of the cooling case of dependent spectrum. After terminating the rinsing procedure those is heated up cooling case with to 40K/s. The held back connections are set free suddenly thereby and supplied to the gas chromatograph directly. This methodology possesses a by far better detector response as the classical Headspace Technik.Bilder, tables and cross references finds you in the attached pdf-DokumentLiteraturverzeichnis1. PH. Eur. European Pharmacopeia, 7th edition. Strasbourg: European Directorate for the quality OF Medicines and Health Care, 2010.2. Bogosian, Gregg and Bourneuf, Edward Vth A of matte OF bacterial life and death. EMBO. 2001, Bd. 2, 9, P. 770-774.3. Eith, Claudia, et al. [Buchverf.] Dock Henning Viehweger. Practical course of the ion chromatography. 2. Edition. Herisau: Metrohm AG, 2007.4. Kohlrausch, Friedrich. Small manual of practical physics. 1. Edition Leipzig: Teubner publishing house, 1870.5. Debye, P. and H�ckel, E. for the theory of the electrolytes. I. Freezing point depression. Physical magazine. Leipzig: S. Hirzel publishing house, 1923. 24, P. 185-206.6. ISO 3696. Water for analytical laboratory use; specification and test methods. Berlin: Beuth publishing house, 1987.7. Internationally Technology Roadmap for Semiconductors. Yield Enhancement. s.l. : www.irts.net, 2003.8. ASTM D1193-06. Standard Specification for Reagent Water. West Conshohocken: ASTM international, Americal Society for Testing and of material, 2011.9. Sheet contactor, German, et al. Advanced detection techniques in the ion chromatography. Herisau: Metrohm AG, 2006.
This text was translated automatically.