Analysis of unwanted particles in parenteral medicines
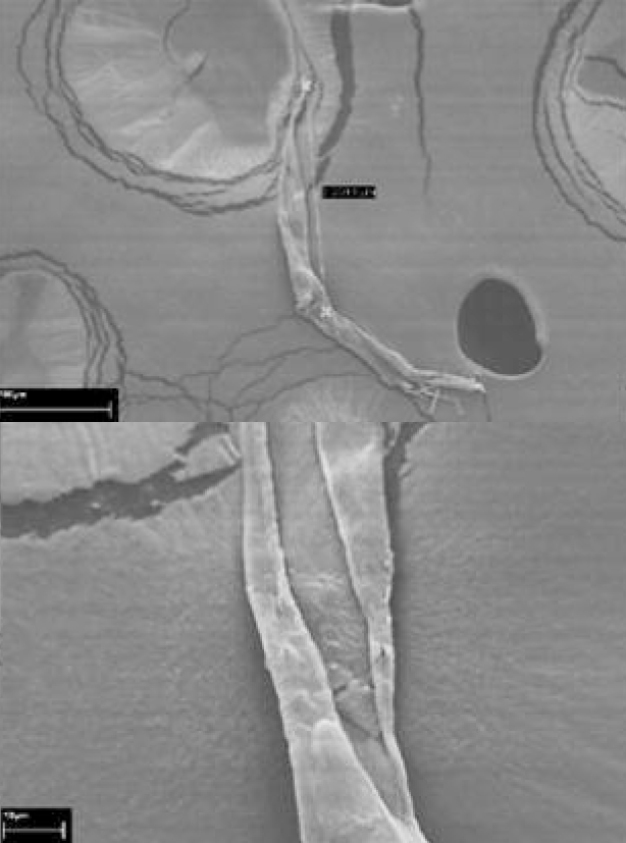
Intravenously applizierte particles represent today still a medical risk, so that they moved in the last years into the focus of the Pharmaforschung. The department of Reinst and micro production at the Fraunhofer IPA developed a method to the analysis of particles in parenteral medicine solutions. With this procedure each particle can be assigned to a material class or a certain material. Injection and infusion solutions, which are given directly to the body by avoidance the gastro-intestinal tract, parenteral medicine solutions so called, can contain both intrinsische and extrinsische particles. Examples of extrinsische, from the outside coming particles, are fibers, glass and abrasion of machine parts. For intrinsische, by the medicine coming particles, can be called active substance precipitations, interactions between prescription components and primary packaging means or, in particular with antibodies with an identical molecular structure (monoclonal), related substances with higher relative molecule mass (aggregates). For the non-visible particles clear limit values and analysis methods are descriptive for investigation in the pharmacopeias. The medical risk, which proceeds from intravenously applizierten particles, is still subject to numerous research work. Here in particular the aggregates and their ability are to cause Immunisierungsreaktionen with the patient to have and/or toxic characteristics into the focus of the Pharmaforschung moved in the last years. Also of verb luring of the blood vessels one reported; the particles are mostly stored however by the body in the fabric (e.g. at the place of the injection). A method to the analysis of particles in parenteral medicine solutions, established at the Fraunhofer IPA, been based on the filtration of a parenteral solution and the analysis on the filter particle present under pure space conditions of the class ISO 1. for a qualitative and quantitative analysis of the filtered particles was used in the abbey lung the Reinst and micro per duktion existing fully automated system rasters of the electron spectroscope (REM), combined with the energiedispersiven X-Ray Spek tro skopie (EDX). This system made possible not only an automated out count all on the filter existing particle, but also for each particle a EDX spectrum. By the comparison of the spectra with a spectrum data base each particle can be assigned to a material class or even a certain material. Thus aimed the pin in heap of hay can be looked for by reading in well-known reference contamination, speaks for example individual particle due to an active substance precipitation under thousands of sodium chloride crystals, which can develop during the filter drying process. An example of the research results, which are based on this methodology, DIAGNOSTICs GmbH of publishing rem pictures of an examined contamination became in pharmind the 5-2011 in co-operation with the company Roche�
This text was translated automatically.
Fraunhofer-Gesellschaft zur Förderung der angewandten Forschung e.V.
80686 München
Germany