- Training, Education
Key cornerstones for quality-compliant production of cleanroom products
What is important when it comes to complying with quality standards for cleanroom products? The experts from the Cleanroom Academy provide the answers.
The manufacture of mostly sensitive, highly complex and/or high-quality cleanroom products in a controlled environment that is as clean as possible, requires specific principles to ensure the desired product properties. After all, cleanroom products are subject to the most stringent requirements.
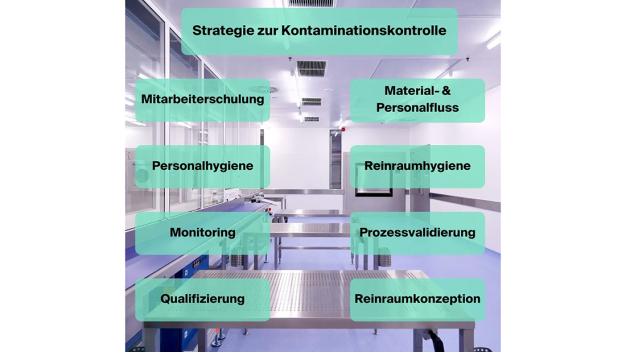
By ensuring that certain fundamentals are in place, companies can guarantee high-quality production of cleanroom products. The following diagram illustrates the most important of these cornerstones:

The contamination control strategy (CCS) forms the basis for all cleanroom decisions and always follows a risk-based approach. To minimise contamination in the clean area, the cleanroom operator must adhere to strict specifications for interiors, maintenance of technical production equipment, as well as for personnel and cleanroom hygiene, for example. This also includes a zone concept that is embedded in the company's building plan. Quality Assurance oversees creating and adhering to the contamination control strategy.
Component Employee Training
Regulartraining of cleanroom personnel on personal hygiene, correct behaviour at the workplace and other cleanroom-specific topics forms the basis for quality-compliant production of cleanroom products. In the end, it serves productivity, compliance with product specifications, and thus the profitability of the entire company.
Both the cleanroom operator on the administrative side and each individual person involved in operational activities must fulfil a certain duty of care in the controlled area. However, personnel can only ever work as well as their level of knowledge and ability allows, and they are motivated. The influence of manufacturing personnel is particularly great in the case of cleanroom products, as humans are usually the greatest source of contamination.
Comprehensive training for cleanroom personnel is not only about conveying and deepening cleanroom-specific knowledge, but also about training the necessary practical skills. For example, the correct gowning, conduct, wiping techniques for cloths and mops or hand hygiene steps must be practised. Knowledge and skills are therefore both required in the cleanroom. Accordingly, the training methods should be customised to the respective topic.
As there are many requirements and prohibitions in the cleanroom area, which staff often find inconvenient, it is a good idea to provide training that is as practical as possible. This means including explanations for the requirements and prohibitions based on the aspects listed in the figure above. If there is a sound understanding of do’s and don’ts then staff will benefit when it comes to cleanroom-compliant implementation, which ultimately has a positive impact on product quality. After all, the company's success will only maximise if everyone involved consistently adheres to the requirements. In contrast, the argument ‘we've always done it this way’ means a standstill and/or can lead to losses in product quality and customer satisfaction in the long term.
Professional external providers such as the CWS Cleanroom Academy offer such training courses. On the one hand, this minimises in-house training efforts. On the other hand, the use of an external expert is usually evaluated differently by cleanroom personnel than if training courses are held with in-house personnel resources. Furthermore, external training offers the opportunity to find out about the state of the art in science and technology in the clean area and to prevent ‘operational blindness’. This requires an inspection of the clean area and the issuing of company standard operating procedures (SOPs) to or by the external expert.
Component Cleanroom Concept
The cleanroom design is crucial to ensure proper operation. A common term in this context is suitability. One also speaks of hygiene design. Surfaces, materials, equipment, facilities and processes should be suitable. What exactly does this mean?
Depending on the product requirements, it is necessary to determine the cleanroom class. This in turn determines the permissible concentration of particles and, if applicable, microorganisms in the air. The design of rooms has an influence on the efficiency of production. Smooth, abrasion-resistant surfaces and easy-to-clean materials and machines are used to minimise contamination and facilitate thorough cleaning and, if necessary, disinfection.
Component Cleanroom Hygiene
In the process landscape of a cleanroom company, the entire process management depends not only on the product, but on operational and cleanroom hygiene. Yet not everything is always gone with a wipe! The first step is to determine the contamination profile. There is also a difference between cleaning and disinfection. Annex 1 of the EU GMP guidelines explicitly addresses this in section 4.22 and states that cleanroom cleaning and disinfection should be defined and controlled as separate processes. Any residues should first be removed before the next cleaning step can be initiated. In practice, these processes are often given too little importance and therefore too little time, as only executors are aware of how time-consuming this work actually is. The importance and therefore value of this effort must be recognised. Furthermore, thoroughly trained and skilled personnel are required in this respect. The cleaning, disinfection and sterilisation measures are always in the area of conflict between cost and benefit or risk for the end user/patient. CWS Cleanrooms and its experts also offer professional advice and service for cleaning and disinfection.
Component Material and Personnel flow
The flow of materials and personnel must be carefully regulated to avoid cross-contamination. Lock systems and special procedures can help to achieve this. In principle, material and personnel flows should be organised in such a way that they primarily serve to protect the product. The shortest possible routes support production and are also economical. To optimise the material flow, the number of people in the cleanroom must be defined. The respective routes should be marked in the layout of the cleanroom system to be able to map the key functions of a safe production process.
Component Monitoring, Qualification and Process Validation
Regular monitoring, qualification and process validation are essential. This also applies to conditions within the cleanroom, including the following room climate parameters: temperature, relative humidity, differential pressure and air quality. The aim of qualification is to provide systematic and documented proof of suitability and fulfilment of the specified performance criteria. In other words, the suitability for the requirements of the clean area needs to be checked and proven, supported by a risk analysis. Qualification is only one part of validation and focuses on the hardware and the environment.
Validation, on the other hand, is the more comprehensive process that ensures that a product or process consistently delivers the expected results. It includes the qualification of equipment and systems as well as process validation. In the latter, the processes themselves undergo validation to show that they function consistently and reproducibly within defined specifications and quality attributes.
Component Monitoring, Qualification and Process Validation
To guarantee the cleanliness class of the cleanroom, personal hygiene is a major factor. Internal rules of behaviour and clothing need to be adhered to strictly. To achieve this, only appropriately trained personnel may enter the clean area. Risk-based measures are defined in the SOPs based on the DIN EN ISO 14644 standard applicable to the cleanroom operator, VDI Guideline 2083 or EU GMP Guideline, Annex 1 and the specific conditions of the respective clean area.
The CWS Cleanroom Academy offers customised, didactically coherent training and consulting solutions on all aspects of cleanrooms and hygiene. Every training course can include various assessments (written and / or practical).
Methodologically, there is an alternation of lessons, working groups, videos and practical exercises, with each participant receiving a handout and a training certificate. Whether in-house at your premises, at the CWS Cleanroom Academy training centres (Dreieich, Leipzig and Marburg) or online.
![]()
Reinraum Akademie
Teil von CWS Cleanrooms
Rosa-Luxemburg-Straße 12-14
04103 Leipzig
Germany
Phone: +49 341 989890
Fax: +49 341 989892108
email: reinraum-akademie@cws.com
Internet: http://www.cws.com/reinraumschulung